Metal nanoparticles synthesis and characterization constitute a major research area in Materials Chemistry. Especially relevant is the use of nanoparticles in nanotechnology applications, because when metal particle size reaches the nanometer scale often shows emerging optical, magnetic, electrical and chemical (catalytic) properties.
The use of fine metal particles in catalysis is especially relevant, because of their irregular surface – which is continuously reconstructed to adapt to the adsorbate -, and their size-dependant electronic properties, which can be tuned to optimize catalytic activity and selectivity. Our group is able to synthesize a wide variety of metal nanoparticles and metal oxide nanoparticles for applications in catalysts, food, energy, etc.
A main challenge in the preparation of nanoparticle colloidal suspensions is stability, since they have the tendency to aggregate to minimize surface tension. For this reason, controlling both the nucleation and growth processes is critical to prepare monodisperse stable colloidal suspensions. In this context, there are several pathways to synthesize nanoparticles including chemical methods, metal evaporation, laser pyrolysis, gas phase methods and plasma-chemical reduction method.
We have been successfully prepared monodispersed sub-nanomatric Pd particles which are first prepared in toluene (organosol), and, if desired, quantitatively transferred to the aqueous phase (see Figure 1). The interest of this method is that allows for obtaining both aqueous solutions with a high concentration of monodispersed Pd nanoparticles (44 mM) and stable suspensions during long periods of time (months). A systematic study has been carried out to master key preparation parameters such as reactants and capping agent concentrations, water content, and stirring rate. Figure 2 shows a TEM micrograph of Pd nanoparticles (from the organosol) and its histogram with a maximum below 1 nm
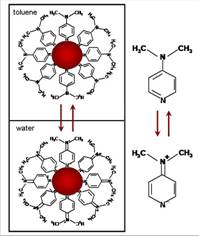
Figure 1. Scheme of the experimental procedure used to transfer the Pd nanoparticles prepared in toluene into the aqueous phase. The phase transfer agent, dimethylaminopyridine (DMAP), neutral in the organic phase, is able to quantitatively extract the nanoparticles into the aqueous phase, where it is present as its zwitterion tautomeric form.
.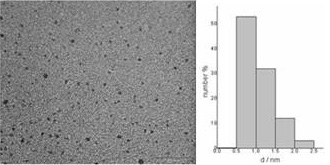
Figure 1. TEM micrograph of Pd nanoparticles in toluene and its corresponding particle size histogram
The controlled reduction of [PdCl4]2- with NaBH4 in toluene produces monodisperse Pd nanoparticles with narrow particle size distribution. Dimethylammonium pyridine (DMAP) was used to smartly transfer Pd to the aqueous phase and to prevent nanoparticle agglomeration. The transfer agent, DMAP, neutral in the organic phase, quantitatively extracts the nanoparticles to the aqueous phase, where it is present as its zwiterionic form (see Scheme at the top of the page).
The colloidal aqueous solution so prepared (Pd hydrosol) is stable for periods of time (over 2 months) even at high nanoparticle concentration (44 mM) without agglomeration. Figure 2 shows a TEM micrograph of Pd nanoparticles (from the hydrosol) with a particle size around 1 nm.
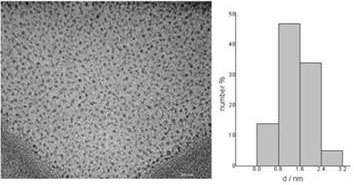
Figure 2. TEM micrograph of Pd nanoparticles in aqueous phase and its corresponding particle size histogram.
Moreover, particle size can be tunning by varying the synthesis conditions thus obtaining particle sizes from 1 to 3.5 nm (Figure 3). Interestingly, magnetic characterization indicated that the larger, 3.5 nm nanoparticles show ferromagnetic properties at room temperature, while the sub-nanometric ones lose this magnetic behavior.
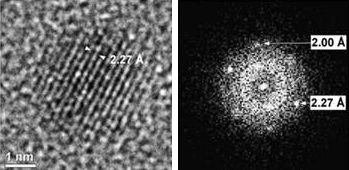
Figure 3. (Left) HRTEM image showing the fringes of the Pd nanoparticles (particle size around 3.5 nm).
(Right) Fast Fourier transform revealing the lattice periodicities of the Pd nanoparticles.
References:
- E. Coronado, A. Ribera, J. Garcia-Martinez, N. Linares, M. Liz-Marzan, Synthesis, characterization and magnetism of monodispersed water soluble palladium nanoparticles, J. Mater. Chem. (18) 5682-5688 (2008)
- J. García-Martínez, N. Linares, S. Sinilbaldi, E. Coronado, A. Ribera, INCORPORATION OF Pd NANOPARTICLES IN MESOTRUCTURED SILICA, Microp. Mesop. Mater. (117) 170–177 (2009)