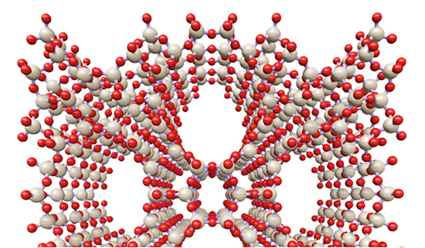
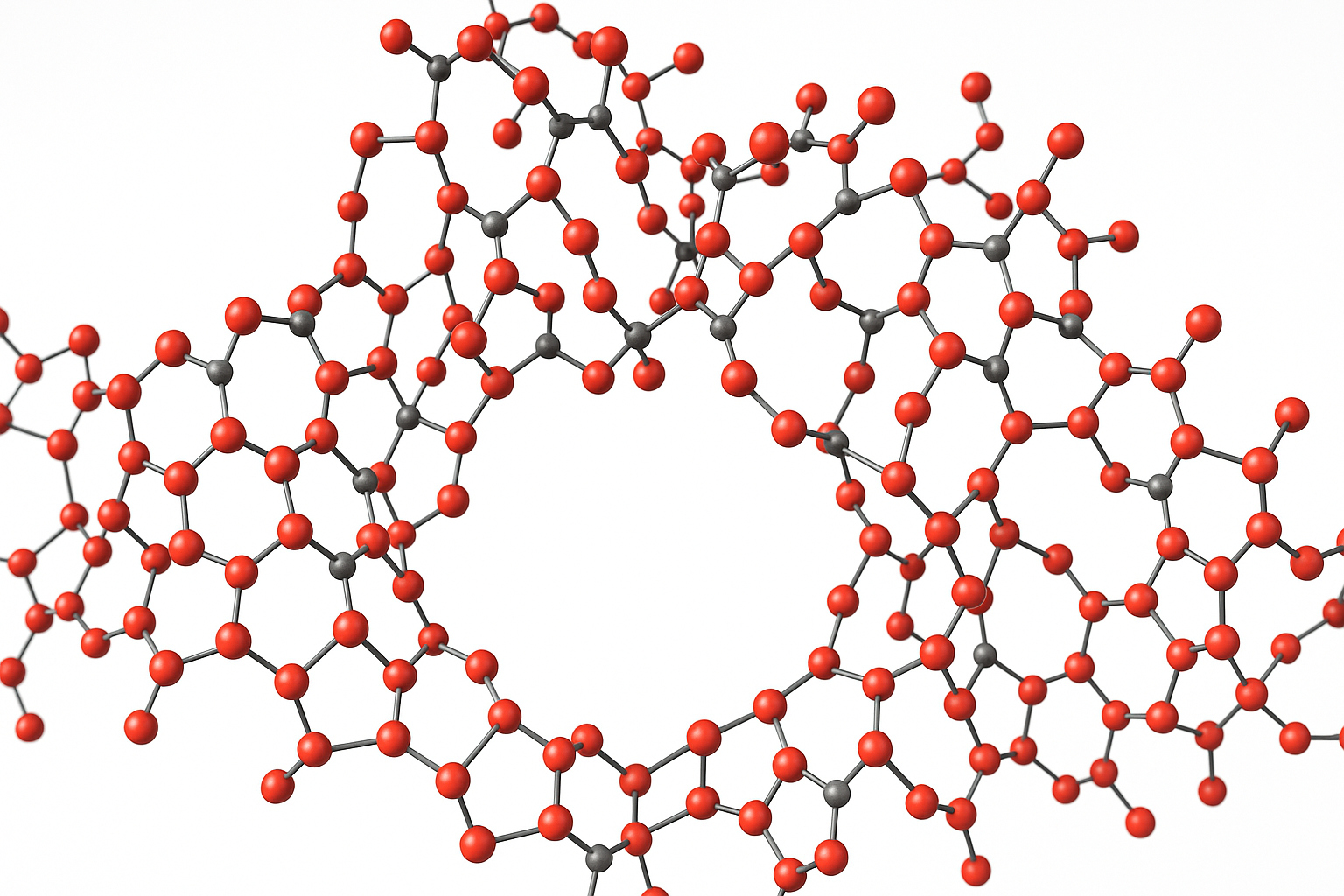
Sol-gel Coordination Chemistry is based on the use of a coordination complex with ligands containing terminal trialcoxysilane groups, which can be hydrolyzed with or without presence of other silica source, to produce a coordination complex-silica network (Figures 1 and 2).
This is a novel synthetic approach based on the hydrolysis and co-condensation of metal alcoxydes linked to metal complexes to prepare hybrid materials with controlled mesoporosity.
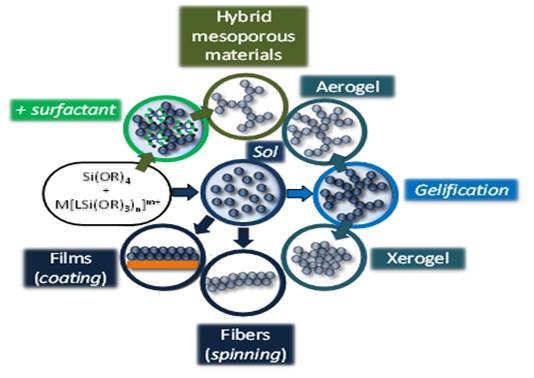
Figure 1. Sol-gel coordination chemistry approach developed for the in-situ incorporation of chemical functionalities into the framework of silica and organosilica porous materials. Photograph show the transparency of an aerogel obtained using a Fe(III)-APTS complex and TEOS as silica precursors (TEOS = tetraethoxysilane, APTS = aminopropyltriethoxysilane).
This method yields novel Mesoporous Metal Complex-Silica (see Figures 1 and 2), with the functionality integrated in the structure of the hybrid material, enhancing at the same time the stability of the coordination complex, which is protected by the silica network [1,2]. Using this strategy, we have prepared a wide range of silica-based hybrid materials including luminescent solids [3] and heterogeneous catalysts [4,5].
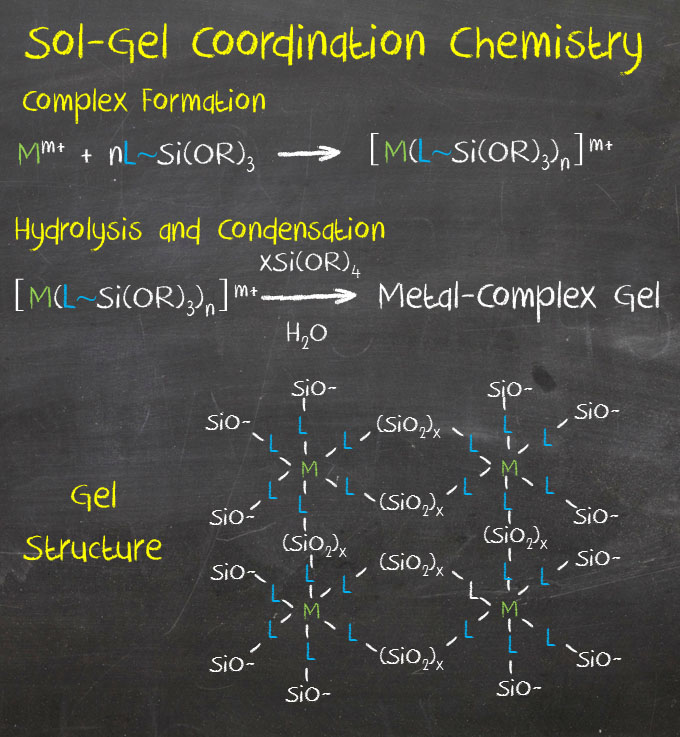
Figure 2. Synthesis of Mesoporous Metal Complex-Silica through the sol-gel coordination chemistry approach.
When a surfactant is used during the synthesis, this method yields novel Mesoporous Metal Complex-Silica (see Figure 3), with the functionality integrated in the structure of the hybrid material, enhancing at the same time the stability of the coordination complex, which is protected by the silica network. Using this strategy, we have prepared a wide range of silica-based hybrid materials including luminescent solids and heterogeneous catalysts.
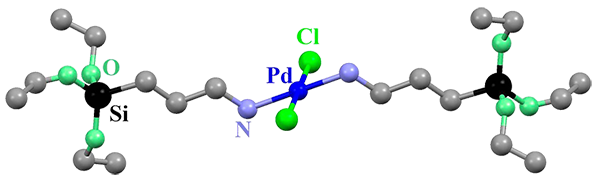
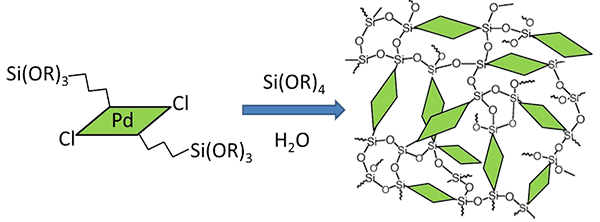
Figure 3. (Up) X-ray molecular structure of the complex [trans-PdCl2(APTS)2] (L=APTS (aminopropyltriethoxysilane). (Bottom) Sol-gel chemistry approach for the in-situ incorporation of this complex into the framework of a mesoporous silica via the covalent bonding of these terminal trialcoxysilane groups with the silica precursor.
This methodology allows for the incorporation not only of coordination, but also a wide range of chemical functionalities such as Pd(0) nanoparticles and Mo cubanes (Figure 4). In addition to these materials, we have incorporated organic moieties in the structure of our hybrid materials containing metal complexes.
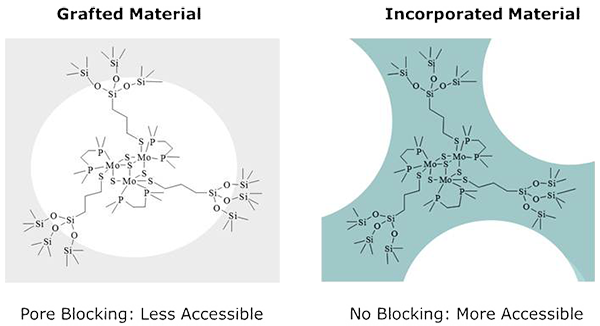
Figure 4. Schematic representation of the Mo siloxo cluster grafted to the pre-existing support (left) in comparison to the in-situ incorporation during the mesoporous silica synthesis thorugh the Sol-gel Coordination Chemistry approach (right).
The versatility of the proposed methodology thus allow for the synthesis of mesoporous materials using cationic, neutral or anionic surfactants, with unique properties derived from the possibility to incorporate not only cationic or neutral metal complexes, but also almost any chemical moiety that could be functionalized with terminal alcoxysilanes.
The proposed sol-gel chemistry approach can be also extended to a wide range of applications ranging from catalysis to biomedical or photonic application, due to the stability of the incorporated functionality (Figure 4).
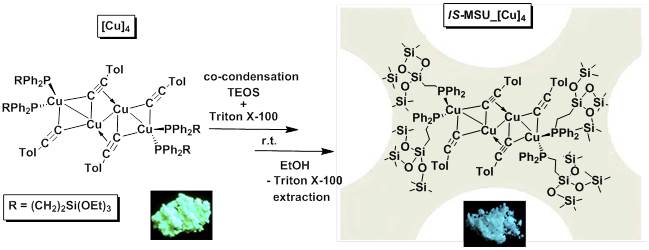

Figure 5. Sol-gel chemistry approach for the synthesis of stable luminescent hybrid mesoporous copper complex-silica material at room temperature and neutral pH (IS-MSU_[Cu]4). IS-MSU_[Cu]4 material shows better stability in comparison with the complex alone and after its grafting on the surface of a pre-existing MSU silica.
References
- E. Serrano, E. Serrano, J.R. Berenguer, J. Garcia Martinez.SOL-GEL COORDINATION CHEMISTRY: BUILDING CATALYSTS FROM THE BOTTOM-UP, Chem. Cat. Chem. in press (2013)
- N. Linares, E. Serrano, M. Rico, A.M. Balu, E. Losada, R. Luque, J. Garcia Martinez. INCORPORATION OF CHEMICAL FUNCTIONALITIES IN THE FRAMEWORK OF MESOPOROUS SILICA, Feature Article, Chem. Commun. (42) 9024–9035 (2011)
- M. Rico, A.E. Sepúlveda, S. Ruiz, E. Serrano, J.R. Berenguer, E. Lalinde, J. Garcia-Martinez. A STABLE LUMINESCENT HYBRID MESOPOROUS COPPER COMPLEX-SILICA. Chem. Commun.(48) 8883–8885 (2012)
- N. Linares, A.E. Sepulveda, M.C. Pacheco, J.R. Berenguer, E. Lalinde, C. Najera, J. Garcia-Martinez. SYNTHESIS OF MESOPOROUS METAL COMPLEX-SILICA MATERIALS AND THEIR USE AS SOLVENT-FREE CATALYSTS, New J. Chem. (35) 225–234 (2011)
- N. Linares, E. Serrano, A.I. Carrillo, J. Garcia-Martinez, METAL-COMPLEX IONOSILICAS: CATIONIC MESOPORUS SILICA WITH NI(II) AND CU(II) COMPLEXES IN THEIR FRAMEWORK, Mater. Lett. (95) 93-96 (2013)
- J. García-Martínez, N. Linares, S. Sinilbaldi, E. Coronado, A. Ribera, INCORPORATION OF PD NANOPARTICLES IN MESOTRUCTURED SILICA, Microp. Mesop. Mater. (117) 170–177 (2009)
- A.I. Carrillo, J. García Martínez, R. Llusar, E. Serrano, I. Sorribes, C. Vicent, A. Vidal-Moya. INCORPORATION OF CUBANE-TYPE MO3S4 MOLYBDENUM CLUSTER SULFIDES IN THE FRAMEWORK OF MESOPOROUS SILICA, Micropor. Mesopor. Mater., 2012, 151, 380
- N. Linares, A.E. Sepulveda, J.R. Berenguer, E. Lalinde, J. Garcia-Martinez, MESOPOROUS ORGANOSILICAS WITH Pd(II) COMPLEXES IN THEIR FRAMEWORK, Microp. Mesop. Mater. (158) 300–308 (2012)