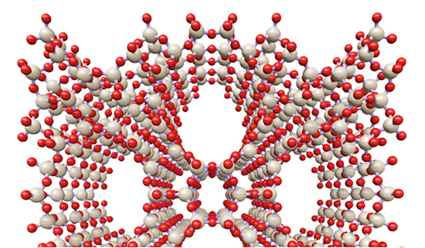
Zeolites are widely used in the chemical industry due to their unique combination of open crystallin structure, ion-exchange capacity, and strong acidity. Their application is limited, however, by the small size of their pores (typically below 0.7 nm).
We have managed to introduce controlled mesoporosity in conventional zeolites by surfactant-assisted crystal rearrangement, dramatically increasing their accessibility to bulky molecules. These materials present enhanced hydrothermal stability over mesoporous silica due their crystalline structure. Recently, we carried out the scale-up and use of mesostrutured zeolite Y on an industrial scale are described, which constitute the first commercial application of hierarchical zeolites.
The incorporation of mesoporosity is done by hydrothermal treatment in the presence of a surfactant (CTAB). Unlike previous attempts, the pH is mildly basic, enough to arrange the framework to accommodate the surfactant but without causing the dissolution of the zeolite (see Figure 1). No silicon or aluminum was detected in the mother liquor. The crystal shape, characteristic of the zeolite, is unchanged during the process. No amorphous phase was observed under the electron microscope. On the contrary, mesoporosity is homogeneously distributed throughout the whole zeolite crystal, as shown in Figures 2-3.
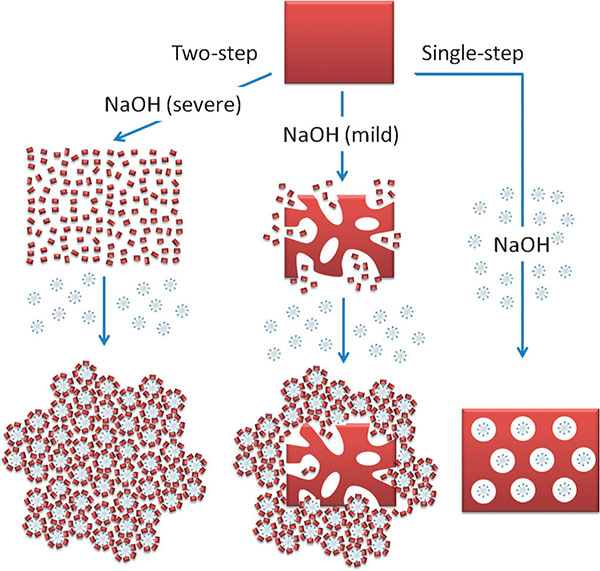
Figure 1. Schematic representation of the differences between the materials prepared by using single-step and two-step approaches with cationic surfactants and NaOH; blue “snowflakes” represent the surfactant micelles, large red rectangle represents a zeolite crystal, small red squares represent silicate/aluminosilicate species that may contain zeolitic subunits.
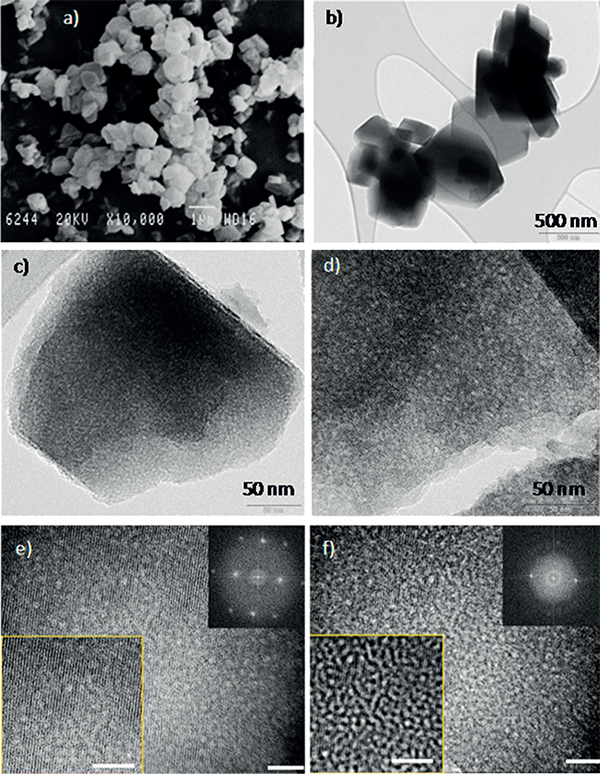
Figure 2. a) SEM and b–f) TEM images of the mesostructured Y zeolites. In (c), a single crystal of mesostructured zeolite shows crystalline lattice fringes and mesoporosity (holes) and, in (d), an ultramicrotomed mesostructured zeolite crystal shows both crystallinity and mesoporosity. e, f) Two TEM images of the same area of a mesostructured zeolite as obtained at two different foci to better visualize the two features of this material, that is, crystallinity and mesoporosity. Scale bars: a) 1 μm, b) 500 nm, c, d) 50 nm, e) 20 nm, f) 20 nm.
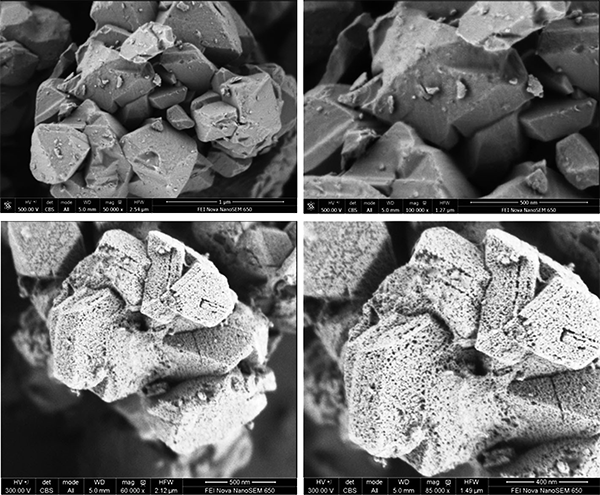
Figure 3. FE-SEM images of the untreated NaY zeolite (top) and the mesostructured Y-zeolite crystals (bottom). Scale bars: a) 1 μm, b, c) 500 nm, d) 200 nm.
These materials have been tested against a FCC catalyst that was made from conventional USY and deactivated under the same conditions for the catalytic cracking of vacuum gas oil (VGO) in an Advanced Catalyst Evaluation (ACE) test unit. Compared to the unmodified zeolite, higher yields of the valuable transportation fuels (gasoline and diesel) were obtained by using the FCC catalyst with the mesostructured USY zeolite although the yields of undesirable coke and unconverted bottoms were reduced (Figure 4).
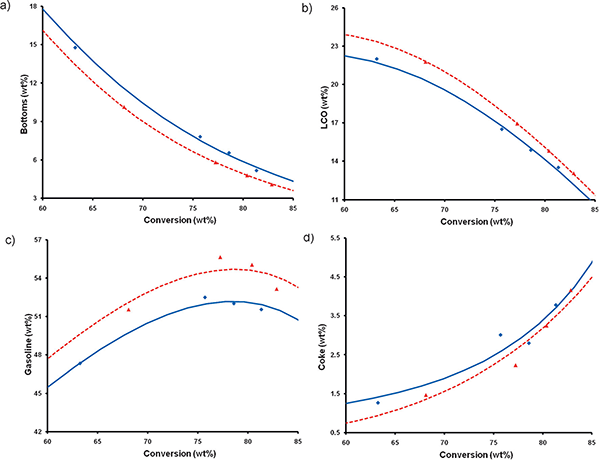
Figure 4. Catalyst evaluation of two FCC catalysts that were prepared and deactivated under the same conditions (788 ºC in 100% steam for 8 h), one of which contained a conventional zeolite USY (blue), whereas the other contained a mesostructured zeolite USY (red). The catalyst evaluation was performed in an ACE unit at 527ºC by using a VGO feedstock. The lines were fitted by a kinetic lump model.
The zeolite-modification process has been scaled up to the commercial scale. FCC catalysts have also been manufactured on the commercial scale, supplied to two separate commercial refineries, and have undergone successful trials that delivered significantly improved product yields and increased economic value to the refiners.
The mesostructured zeolite Y was able to: 1) Increase the yields of gasoline and diesel [light cycle oil (LCO)]; 2) increase the production of valuable light olefins (propylene and butenes); and 3) remarkably decrease the yield of coke. Most importantly, these advantages were associated with almost no penalty in catalyst activity (as evidenced by the very close catalyst-to-oil ratios that were necessary to achieve 75% conversion). The estimated economic uplift based on the laboratory test results was about $2.00/barrel of FCC feed for the specific refinery. At the end of the commercial trial, the additional revenue that was delivered to the refinery by replacing the incumbent catalyst with the FCC catalyst that contained the new mesostructured zeolite Y, was estimated to be over $2.50/bbl of the FCC feed (Figure 5).
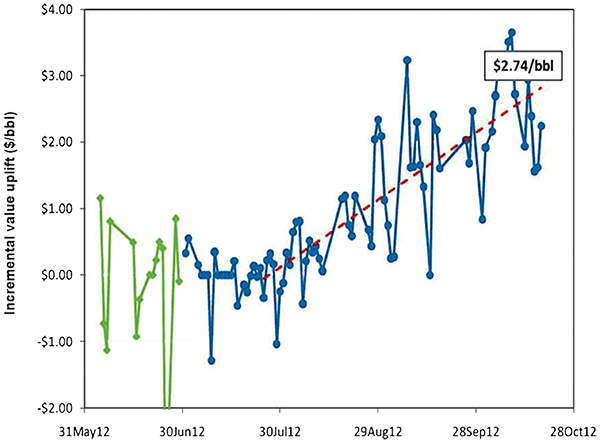
Figure 5. Observed trend during a trial at Alon’s Big Spring, Texas refinery. An incremental value uplift owing to the change-out of the incumbent catalyst for Rive’s MH-1 catalyst that contained mesostructured Y zeolite.
Rive Technology began the ongoing supply of the very first commercial FCC catalyst with surfactant-templated mesoporous zeolite Y in early April 2013. The era of mesostructured zeolites in industrial catalysis has arrived!
References
- J. García-Martínez, K. Li, G. Krishnaiah, A mesostructured Y zeolite as a superior catalyst – from lab to refinery. Chem. Commun. (48) 11841-11843 (2012)
- J. García-Martínez, M. Johnson, J. Valla, K. Li, J.Y. Ying, Mesostructured zeolite Y—high hydrothermal stability and superior FCC catalytic performance, Catal. Sci. Technol. (2) 987–994 (2012)
- K. Li, J. Valla, J. Garcia-Martinez, Realizing the commercial potential of hierarchical zeolites: new oportunities in catalytic cracking, Chem. Cat. Chem. (6) 46–66 (2013).